Published on:
The diabetes drug saxagliptin marketed as Onglyza and Kombiglyze XR) may be a defective drug that increases the risk of hospitalization for heart failure
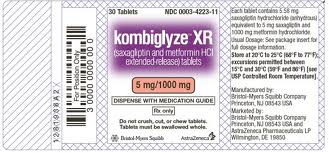 Drug manufacturers are liable for the safety of their products. Mostly for profit reason some trial results may be overlooked and drugs may be launched on the market with dangerous side effects. A recent study published in the New England Journal of Medicine showed that patients with diabetes using the drug saxagliptin had an increased risk to be hospitalized for heart failure.
Drug manufacturers are liable for the safety of their products. Mostly for profit reason some trial results may be overlooked and drugs may be launched on the market with dangerous side effects. A recent study published in the New England Journal of Medicine showed that patients with diabetes using the drug saxagliptin had an increased risk to be hospitalized for heart failure.
For this reason The FDA just published a safety announcement that it requested clinical trial data from the manufacturer of saxagliptin to investigate a possible association between use of the type 2 diabetes drug and heart failure.
Read the Safety Announcement from the FDA
 New York Personal Injury Attorneys Blog
New York Personal Injury Attorneys Blog