Advocating for the Grieving Families Act: A Step Toward Justice and Accountability
Yesterday, our partner Christopher Donadio and his client Jose Marrero were featured on The Rush Hour on NY1 and interviewed by Annika Pergament. The segment highlighted the urgent need for the Grieving Families Act (GFA) and the devastating personal impact of New York’s outdated wrongful death laws. Jose Marrero shared his tragic story of losing his wife during a routine procedure at Maimonides Medical Center, underscoring the emotional toll on families who are left without fair recourse under current legislation.
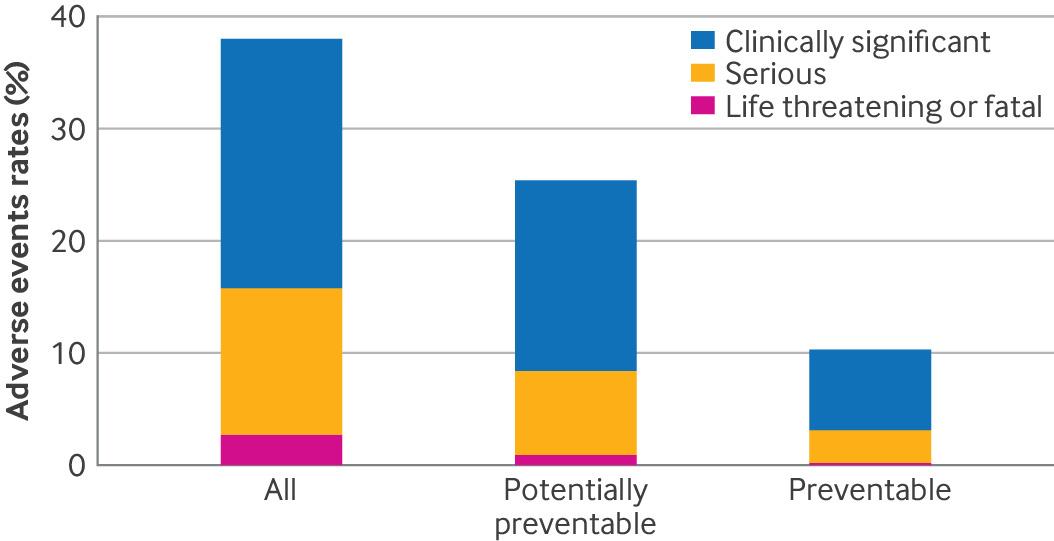
The Grieving Families Act (GFA) aims to rectify one of the most longstanding injustices in New York law—the denial of wrongful death restitution for grief and emotional suffering. Under the current system, compensation is limited to the lost income of the deceased and any pain and suffering prior to death. This outdated approach often leaves families of children, stay-at-home parents, and elderly individuals without any restitution, perpetuating systemic inequities.
 New York Personal Injury Attorneys Blog
New York Personal Injury Attorneys Blog