Articles Tagged with Surgical complication
New Study Reveals High Rates of Preventable Surgical Adverse Events in U.S. Hospitals
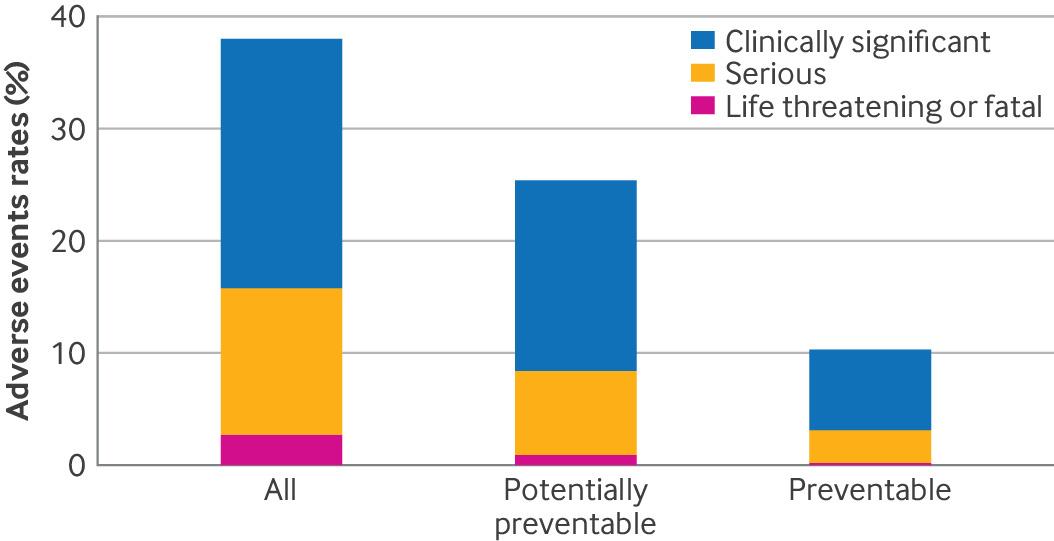 Recent findings published in the British Medical Journal (BMJ) have revealed alarming statistics about the safety of surgical care in hospitals. This extensive study, conducted across 11 U.S. hospitals, highlights the urgent need for improved safety measures to prevent adverse events during surgery. As medical malpractice attorneys, we understand the devastating impact such errors can have on patients and their families, especially when these adverse events are preventable.
Recent findings published in the British Medical Journal (BMJ) have revealed alarming statistics about the safety of surgical care in hospitals. This extensive study, conducted across 11 U.S. hospitals, highlights the urgent need for improved safety measures to prevent adverse events during surgery. As medical malpractice attorneys, we understand the devastating impact such errors can have on patients and their families, especially when these adverse events are preventable.Post surgical complication risks when surgeons use Acellular Dermal Matrix (ADM) in implant-based breast reconstruction

The off-label use of Acellular Dermal Matrix (ADM) products in implant-based breast reconstruction can result in post surgical complications and problems for patients up to two years after the surgery according to a recent analysis by the FDA.
Off-label use not approved by FDA
Accellular Dermal Matrix is a type of surgical mesh that is developed from human or animal skin. It has been approved by the FDA for specific use such as hernia surgery and more recently many surgeons specializing in breast reconstruction after a mastectomy have been using the product off-label. The off-label use of ADM for breast reconstruction has never been approved by the FDA and a recent analysis found that some patients suffered complications up to two years after the surgery.
Surgical malpractice: what are the most common errors and how to avoid them?
 More than 40 million Americans are undergoing surgery every year. An estimated 35.8 million of them will immediately return home after having their surgery performed in a freestanding ambulatory surgery center or in a hospital-based outpatient setting. Another 7 million will be required to stay at the hospital after their surgery. While most patients fully recover from their surgery without problems some of them will suffer from surgical complications or errors. It is estimated that around 14% of surgical patients encounter at least one adverse event.
More than 40 million Americans are undergoing surgery every year. An estimated 35.8 million of them will immediately return home after having their surgery performed in a freestanding ambulatory surgery center or in a hospital-based outpatient setting. Another 7 million will be required to stay at the hospital after their surgery. While most patients fully recover from their surgery without problems some of them will suffer from surgical complications or errors. It is estimated that around 14% of surgical patients encounter at least one adverse event.
In a recent study, the ECRI and the Institute for Safe Medication Practices took a close look at surgical malpractice and analyzed 2,400 surgical adverse events that were recently reported to them. Among these 2,400 reported surgical malpractice events, researchers found that 1,561 of them were relevant. They found that 478 of them (31%) were complications related to the surgery, 460 (29%) of them were adverse events related to patient and operating room readiness, 377 (24%) were retained surgical items, 102 (6.5%) were contaminations, 80 (5.1%) were adverse events caused by equipment failure and 64 (4.1) were wrong surgeries.
To reduce these adverse events, the ECRI recommended the following strategies:
Surgery Centers not mandated to report death or serious injuries in 17 States
Our Medical Malpractice Attorneys Jeffrey Bloom and Ben Rubinowitz represented the family of Joan Rivers after she died during a routine endoscopy at a Manhattan surgery center. Sadly many other patients have died following complications or surgical errors at this type of center as many States still do not have regulations that may prevent them. For example in most of the country there is no law that prohibits a doctor who was laid off by a hospital for misconduct to open a surgery center.
A recent USA TODAY NETWORK and Kaiser Health News investigation found that a surgery center in Arkansas 3 people died during colonoscopy procedures in 15 weeks and none of them were reported to an oversight authority. Patients coming for procedures were obviously not aware of these deaths either. This must change.
The lack of oversight continues to kill patients at surgery centers
Medical Malpractice: Surgical Fire
Most Surgical Fires result from Medical Malpractice or negligence causing serious injury, disfigurement, and even death. They occur in, on or around a patient who is undergoing a surgical procedure. An estimated 550 to 650 surgical fires occur in the United States per year. Despite the fact that the root causes of surgical fires are well-understood, surgical fires still occur.
To promote actions to reduce the risk of risk of surgical fires.The Preventing Surgical Fires Initiative is celebrating its second anniversary during National Fire Prevention Week, October 6-12, 2013.
Fires happen during surgery because the 3 elements needed to start a fire (fire triangle) are present in an operating room:
Medical Malpractice – More complications after minimal invasive procedure to remove kidney stones
Percutaneous nephrolithotomy, or PCNL, is a minimally invasive urological surgery during which a surgeon removes medium to large kidney stones through a small incision in the back using a hollow scope. The use of PCNL is increasing especially among women and complications are on the rise particularly blood infections. Patients are at risk of developing complications if they are older, sicker and treated in more recent years. Age is significantly associated with increased odds of mortality according to a research from from Khurshid R. Ghani, M.D., of Henry Ford Hospital’s Vattikuti Urology Institute, which was published in the Journal of Urology.
 New York Personal Injury Attorneys Blog
New York Personal Injury Attorneys Blog



