Methylene Chloride, a toxic paint stripper, continues to kill and injure workers and consumers
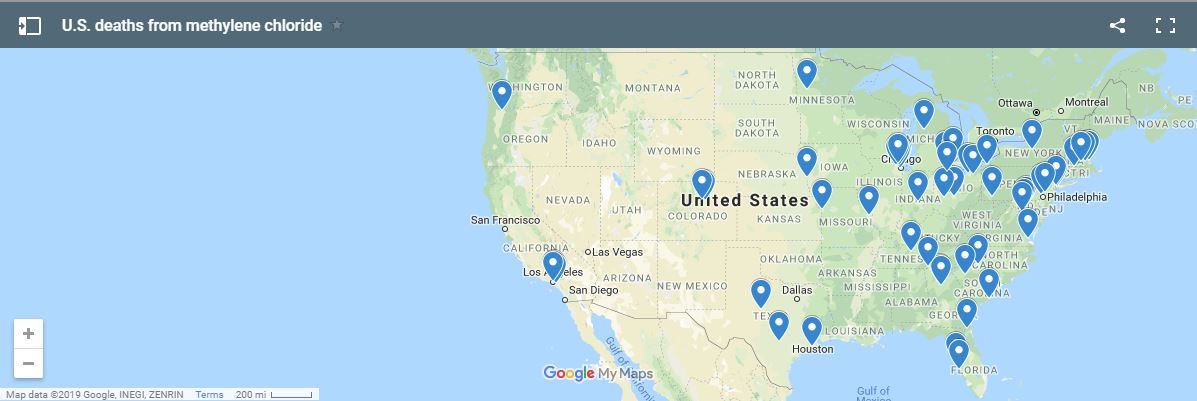 Methylene Chloride is a dangerous product that has previously caused the death of many people. Short term exposure to methylene chloryde can cause mental confusion, nausea, vomiting , headache and death in the worst cases. In the long term, workers exposed to it can develop cancer, nervous system problems, toxicity in their kidney, liver and reproductive system.Major retailers such as Lowes and Home Depot have removed it from their shelves even before the EPA finalized a ban proposal that was published one day before President Obama left office. In Europe the deadly chemical was pulled from general use in 2011.
Methylene Chloride is a dangerous product that has previously caused the death of many people. Short term exposure to methylene chloryde can cause mental confusion, nausea, vomiting , headache and death in the worst cases. In the long term, workers exposed to it can develop cancer, nervous system problems, toxicity in their kidney, liver and reproductive system.Major retailers such as Lowes and Home Depot have removed it from their shelves even before the EPA finalized a ban proposal that was published one day before President Obama left office. In Europe the deadly chemical was pulled from general use in 2011.
Last May, Scott Pruit confirmed that the EPA was committed to finalize the proposed methylene chloride ban however last month the EPA drafted two new final rules that would ban the use of methylene chloryde to regular consumers but not to commercial operators. Two new final rules drafted by the EPA allow the usage of the dangerous product for commercial use as long as the workers using it have been trained. These rules that have not been made public yet but that have been sent by the EPA to the Office of Management and Budget have sparked the furor of public health advocates and and of congressional Democrats. This is a major step back compared to the proposed rule announced by the EPA during the Obama era.
 New York Personal Injury Attorneys Blog
New York Personal Injury Attorneys Blog








 Takata just recalled an additional 2.7 million vehicles that may have a
Takata just recalled an additional 2.7 million vehicles that may have a 