After two patients suffered serious personal injury because they were administered non sterile simulation intravenous fluids at a New York urgent car facility, the NYSDOH and the CDC discovered that simulation medical products had inadvertently entered the clinical supply chain
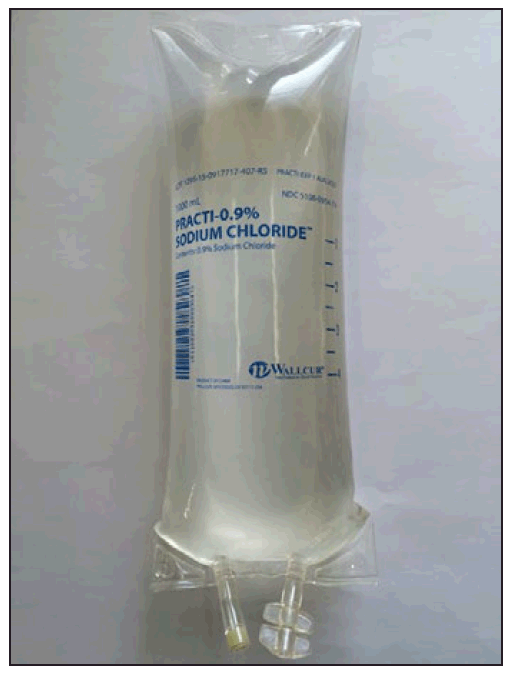 Two patients were seriously injured in a New York urgent care facility after they were inadvertently administered non sterile simulation intravenous fluids. They both experienced a febrile illness during administration and had to be hospitalized. One of them developed sepsis. Both of them survived.
Two patients were seriously injured in a New York urgent care facility after they were inadvertently administered non sterile simulation intravenous fluids. They both experienced a febrile illness during administration and had to be hospitalized. One of them developed sepsis. Both of them survived.
The cases were reported to the New York State Department of Health (NYSDOH)last year. The NYSDOH began a collaborative investigation with the CDC in December. The investigation found that four other New York outpatient facilities had received Wallcur simulation saline. All facilities said they had ordered the real product and weren’t aware that they had received a simulation product until they were were notified by the NYSDOH. Fortunately none of the facilities had used the product yet.
Wallcur recalled all its saline simulation products from the market at the beginning of the year (see previous blog). Investigation was pursued by the CDC at a national level. So far nationally 9 adverse events have been reported for 25 people including 11 hospitalizations. Two deaths occurred even though it wasn’t clear that they were related to the administration of the product. All clinical facilities that received the products confirmed that they were not aware at time of purchase that the product was intended for simulation only. Read more on the CDC website
 New York Personal Injury Attorneys Blog
New York Personal Injury Attorneys Blog