Hospitals sharing multi-dose diabetes pen devices among patients is medical malpractice that the FDA is trying to fight by requiring additional label warnings
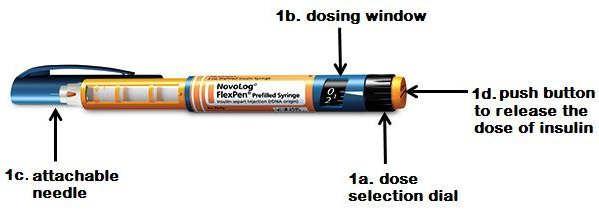
Sharing diabetes pens among patients is a gross medical malpractice that can lead to transmission of infections and viruses such as HIV and hepatitis viruses. According to the FDA, since 2008 thousands of patients may have been exposed to blood-borne pathogens from the sharing of multi-dose pen devices for insulin and other injectable diabetes medicines. In 2009 the FDA was informed that 2,114 patients from a U.S. army facility had been injected with pens that had been used on other patients. Then in 2011, 2,345 patients from the Dean Clinic in Wisconsin were notified that pen and needles had been shared among patients. More recently in 2013, 716 patients from the Veteran Health Administration were notified of potential exposure to infections through the sharing of diabetes pen. Last March in New York, the South Nassau Community Hospital in Long Island contacted 4000 patients to be screened for HIV and Hepatitis after a nurse said she was using the same insulin pen for multiple patients (see previous blog).
Insulin pens and pens for other injectable diabetes medicines should never be shared among patients, even if the needle is changed. To promote safe use, the FDA is requiring that pens and packaging containing multiple doses of insulin and other injectable diabetes medicines display a warning label stating “For single patient use only.” Read the safety announcement from the FDA
 New York Personal Injury Attorneys Blog
New York Personal Injury Attorneys Blog