Published on:
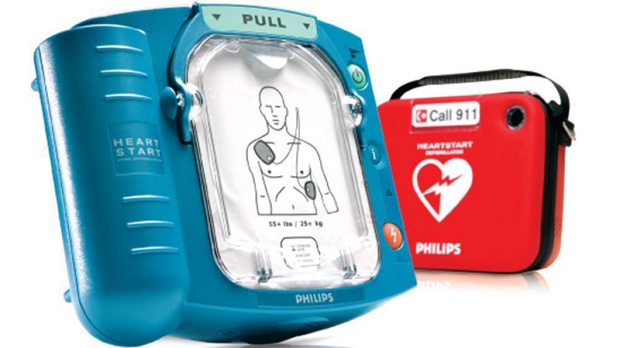
Product Liability – FDA warns that HeartStart Home Defibrillator recalled by Philips may fail to deliver a life-saving shock in a life or death situation
A defective electrical component in Philips HeartStart defibrillators may fail to deliver a needed shock in case of emergency, warned the FDA yesterday. This safety alert by the FDA follows the recall by Philips of three models of its HeartStart devices in September 2012 due to an internal electrical malfunction.
The recall affects about 700,000 defibrillators sold between 2005 and 2012. The FDA recommends Customers who have received the affected devices contact Philips at 1-800-263-3342 to receive a replacement.
 New York Personal Injury Attorneys Blog
New York Personal Injury Attorneys Blog


