Product Liability: Dietary Supplement OxyElite Pro pulled from shelves nationwide after a mother of seven died and two people required liver transplants in Hawaii
1 person died and 11 suffered serious personal injury and were hospitalized with two having to receive liver transplants following multiple non viral Acute Hepatitis cases in Hawaii. Out of a total of 29 cases, 24 were linked to a dietary supplement product labeled as OxyElite Pro.
The CDC is looking at other cases of liver injury nationwide that may be related.
The dietary product OxyElite Pro was pulled out from shelves nationwide and USPlabs LLC of Dallas stopped its distribution.Consumers who have bought this product should stop to use it immediately. Consumers who believe they have been armed by using this product or any other dietary supplement should contact their doctor immediately.
Read the whole story in the Huffington Post
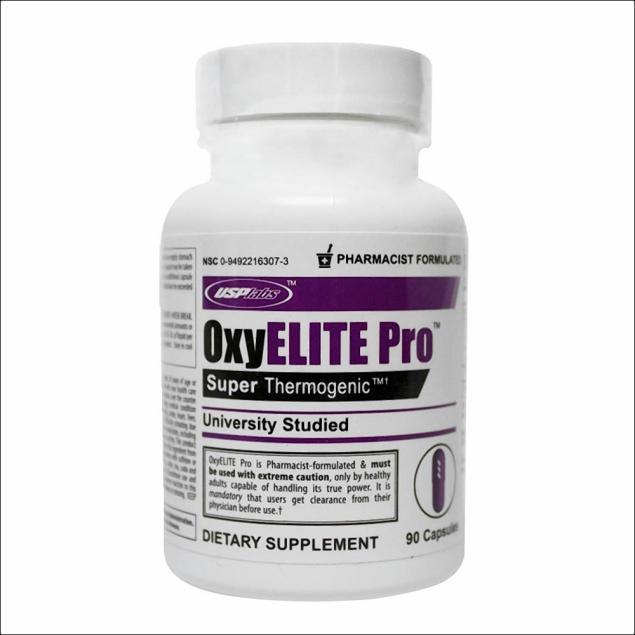
Dietary supplements like OxyElite Pro do not have to be approved by the FDA before they hit shelves.
 New York Personal Injury Attorneys Blog
New York Personal Injury Attorneys Blog


