Falls are the leading cause of death in the construction industry in New York and in the US. Many of those deaths are preventable. To raise awareness of preventing fall hazards, OSHA asked employers in New York and all over the nation to organize during this week a voluntary event to talk to their employees about fall prevention safety.
In New York State, several events already occurred at the beginning of this week. On Monday June 2nd Turner Construction Company suspended work across the company to deliver a message focused on “Proper Pre-Task Planning for Safety” to 45,000 employees. The same day, in Malta, NY, Turner hosted a falls in construction stand down for 700 employees.
On Tuesday, in Manhattan, Related Hudson Yards hosted two falls in construction stand down events along with the sites general contractor, Tutor-Perrini (Buildings) for about 800 employees.
 New York Personal Injury Attorneys Blog
New York Personal Injury Attorneys Blog


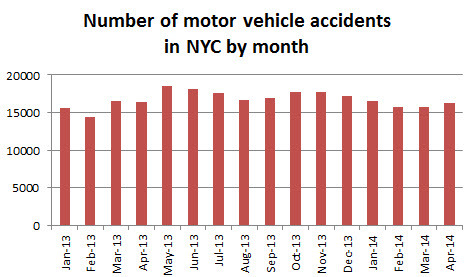
 The long legal battle that ensued after two
The long legal battle that ensued after two 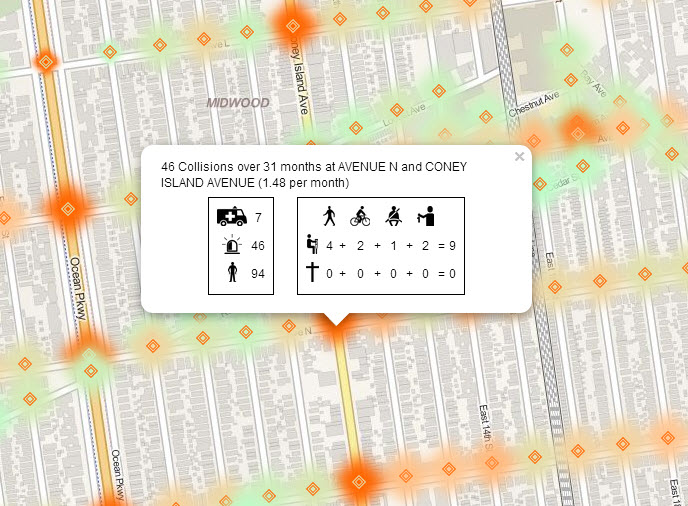 6 hours later a 58 year old man suffered serious personal injury after being struck by a car while crossing Nostrand Ave at Church Ave in Brooklyn as well.
6 hours later a 58 year old man suffered serious personal injury after being struck by a car while crossing Nostrand Ave at Church Ave in Brooklyn as well. 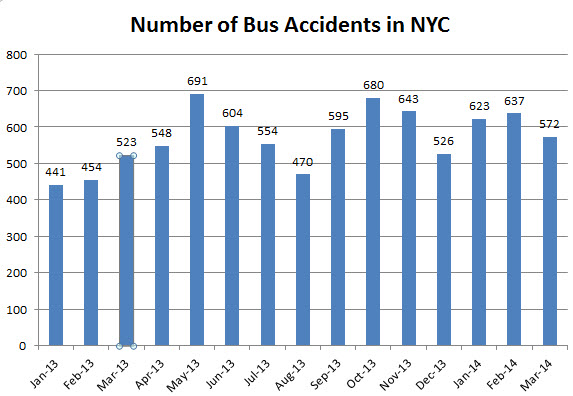
 Previous studies have shown that warning signs for speed cameras help slow down speeding drivers and the city of New York is looking at this option according to the Department of Transportation Commissioner Polly Trottenberg.
Previous studies have shown that warning signs for speed cameras help slow down speeding drivers and the city of New York is looking at this option according to the Department of Transportation Commissioner Polly Trottenberg.
