By Anthony Gair
A. COST
Product liability cases are different from general negligence cases and even medical malpractice cases for several reasons. First and foremost is the cost involved in litigating these cases. The costs involved for the plaintiff’s attorney can be astronomical. It is not unusual to accumulate well over $100,000.00 in expenses prior to trial. Obviously, much more may be expended should the case proceed to trial. Thus, when you, as a plaintiff’s attorney, are considering the representation of an injured plaintiff, the first question that must be answered is whether it is worth the cost, time and resources that will have to be put into the prosecution of the case. It is suggested that, with some exceptions, a case not be undertaken unless it has a value in excess of seven figures. However, if you are dealing with a product with a documented manufacturing flaw or defect which you know has been previously litigated or recalled, the potential value of the case can be less. Similarly, in a design defect case, if there has been a product recall, the same applies. Further, if you have previously successfully litigated a case involving the same product there is no reason not to take it, even if it is not a seven figure case.
The plaintiff’s attorney must also understand that manufacturers are proud of their products and will not simply roll over and play dead. Many manufacturers have defense firms on retainer to handle cases brought against them throughout the country. These attorneys know the product involved, are generally highly experienced and have the best experts at their disposal in addition to the design engineers who designed the product. These national counsel have relationships with local attorneys who also have expertise in defending these cases.
Since most of the cases you will see are those involving alleged design defects and/or failure to warn this article will focus on those types of cases. It is suggested that if the only theory you can come up with is that of failure to warn, unless it is clear cut, you should be cautious about taking the case, since in New York culpable conduct is a defense to a Product Liability case. In practice, if you have a failure to warn case, you will usually have a design defect case as well.
B. TIME AND PUTTING IT TOGETHER
If you undertake to handle a product liability case, be prepared to spend a countless number of hours on it. Not only will you have to learn all there is about the particular product, as discussed further, you must learn the principles of safety design engineering. Similar to a medical malpractice case in which you must learn the area of medicine involved so as to effectively cross-examine the defendant doctors and their experts, the same is true as to the field of design engineering.
The most important first step is to choose the right expert or experts. Under no circumstances pick a generic expert, one that testifies in any type of case. It is imperative that you choose a case specific expert who has actually worked in the industry and will survive a Daubert/Frye preclusion motion.
Continue reading →
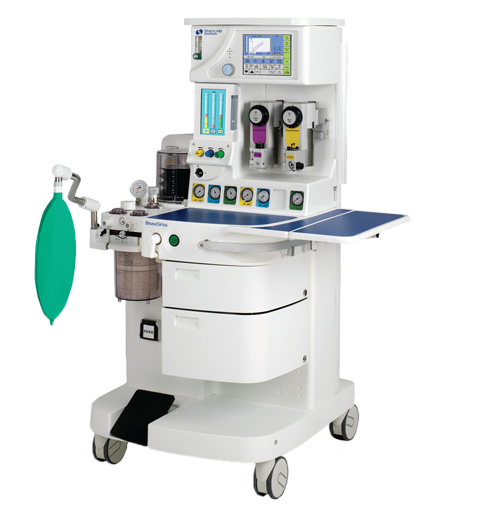 Some potentially defective Anesthesia workstations and service kits manufactured by Spacelabs Helathcare are subject to a class I recall by the FDA. The recalled models are the BleaseSirius Anesthesia Workstation, the BleaseFocus Anesthesia Workstation, and Service Kits Part Number 050-0659-00 and 050-0901-00.
Some potentially defective Anesthesia workstations and service kits manufactured by Spacelabs Helathcare are subject to a class I recall by the FDA. The recalled models are the BleaseSirius Anesthesia Workstation, the BleaseFocus Anesthesia Workstation, and Service Kits Part Number 050-0659-00 and 050-0901-00.  New York Personal Injury Attorneys Blog
New York Personal Injury Attorneys Blog


 Volkswagen is recalling several popular models for the the following reasons:
Volkswagen is recalling several popular models for the the following reasons:
 Fear of possible Listeria Contamination prompted several companies to recall their ready to eat salad kits as the
Fear of possible Listeria Contamination prompted several companies to recall their ready to eat salad kits as the 