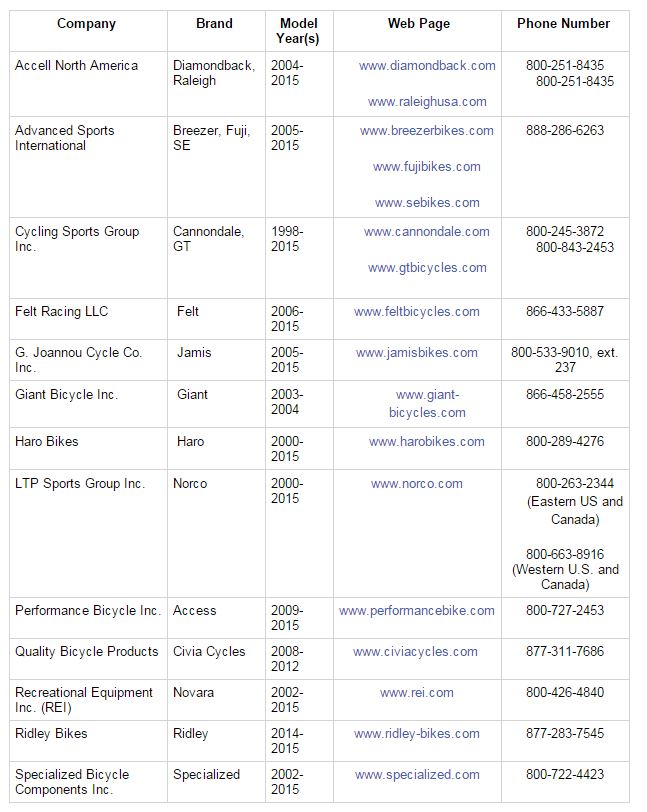
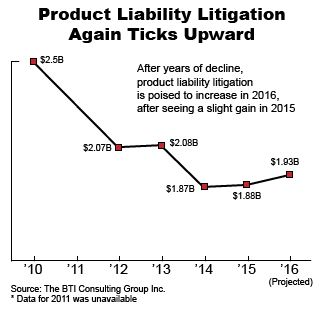
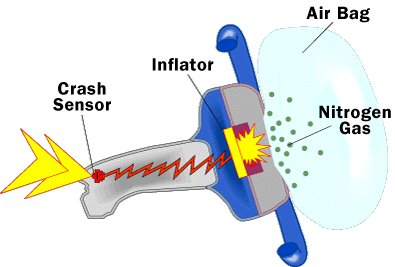
Our managing partner Ben Rubinowitz was recently named New York City “Lawyer of the Year” for product liability litigation – plaintiffs 2016
 Gair, Gair, Conason, Rubinowitz, Bloom, Hershenhorn, Steigman & Mackauf is proud to announce that their managing partner New York Product Liability Lawyer Ben Rubinowitz was recently named New York City “Lawyer of the Year” for product liability litigation – plaintiffs 2016. “There is no substitute for hard work,” says Rubinowitz who explained that he “feels fortunate to work with such an outstanding group of lawyers.” When asked about his success, Rubinowitz shared credit for the results with the attorneys in his firm. “Every one of our lawyers knows that hard work and preparation are the keys to success. There are no shortcuts in this business. Our clients come to us in a time of great need. Many have suffered through life changing events, many have lost family members and many feel that their life has been ruined.” It is against this backdrop that Rubinowitz and the lawyers at his firm go to work. “We approach every case with the same level of scrutiny. Our goal is to obtain justice for our clients. I know of no other firm that puts in the effort that we do.”
Gair, Gair, Conason, Rubinowitz, Bloom, Hershenhorn, Steigman & Mackauf is proud to announce that their managing partner New York Product Liability Lawyer Ben Rubinowitz was recently named New York City “Lawyer of the Year” for product liability litigation – plaintiffs 2016. “There is no substitute for hard work,” says Rubinowitz who explained that he “feels fortunate to work with such an outstanding group of lawyers.” When asked about his success, Rubinowitz shared credit for the results with the attorneys in his firm. “Every one of our lawyers knows that hard work and preparation are the keys to success. There are no shortcuts in this business. Our clients come to us in a time of great need. Many have suffered through life changing events, many have lost family members and many feel that their life has been ruined.” It is against this backdrop that Rubinowitz and the lawyers at his firm go to work. “We approach every case with the same level of scrutiny. Our goal is to obtain justice for our clients. I know of no other firm that puts in the effort that we do.”
Read the complete article in Best Lawyers
 New York Personal Injury Attorneys Blog
New York Personal Injury Attorneys Blog