Proper diagnosis of Denys-Drash syndrome
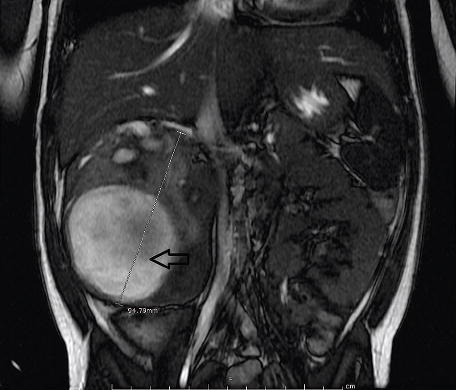
Failure to timely diagnose Denys-Drash syndrome can be medical malpractice that can lead to renal failure and ultimately death. Denys-Drash syndrom is a very rare congenital disorder that affects young children. There are only 150 known cases in the world therefore very little information is available for doctors to diagnose and treat this disorder.
What is known so far is that 90% of the children with this disorder develop a rare pediatric kidney cancer known as Wilms tumor. Undescended testes and severe proximal hypospadias are also associated with this disorder. The Journal of the American Academy of Physician Assistants (JAAPA) recently released an article describing the case of a 3 year old patient affected by this syndrome. The authors Shawn C. Smith, Barry Chang and Laura Beth Fleming are all from the Cardon Children Medical Center in Mesa, AZ where the patient was admitted. The article describe how the authors of the article diagnosed the disorder and which treatments were used to treat the patient. The complete article can be found here
 New York Personal Injury Attorneys Blog
New York Personal Injury Attorneys Blog