A hospital worker at Bronx Montefiore Medical Center sold patients financial information to scammers who went shopping at Barneys and other high end retailers in Manhattan
 More than 12,500 patients of a New York Hospital had their financial records compromised after an assistant clerk sold them for $3 a piece to a ringleader who made purchases at various high end retailers in Manhattan. Between January and June 2013 32 year old Monique Walker who worked in the financial department of the Montefiore Medical Center in the Bronx sold information such as names of patients, social security numbers, credit card numbers and birth dates to 28 year old Fernando Salazar who was sending his “buyers” Patricia Charles, 43, Lawrence Davenport-Brown, 23 and Charde Lawrence, 28, of Staten Island, Ashly Garrett, 25, of Queens, Sasha Rivera, 31, of Brooklyn, and Crystal White, 32 to shop at various high end venues in Manhattan. They all face charges including grand larceny, possession of a forged instrument, identity theft, unlawful possession of personal identification information and related counts. Read more in the NY Daily News
More than 12,500 patients of a New York Hospital had their financial records compromised after an assistant clerk sold them for $3 a piece to a ringleader who made purchases at various high end retailers in Manhattan. Between January and June 2013 32 year old Monique Walker who worked in the financial department of the Montefiore Medical Center in the Bronx sold information such as names of patients, social security numbers, credit card numbers and birth dates to 28 year old Fernando Salazar who was sending his “buyers” Patricia Charles, 43, Lawrence Davenport-Brown, 23 and Charde Lawrence, 28, of Staten Island, Ashly Garrett, 25, of Queens, Sasha Rivera, 31, of Brooklyn, and Crystal White, 32 to shop at various high end venues in Manhattan. They all face charges including grand larceny, possession of a forged instrument, identity theft, unlawful possession of personal identification information and related counts. Read more in the NY Daily News
 New York Personal Injury Attorneys Blog
New York Personal Injury Attorneys Blog



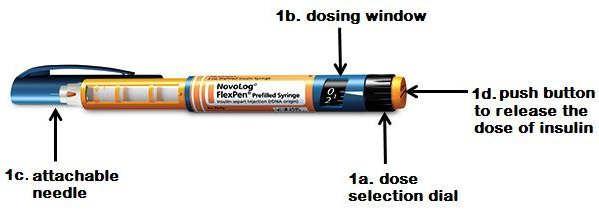
 Device-related hazards can lead to medical malpractice. In its 2015 top 10 Health Technology hazards, ECRI Institute lists 10 safety topics deemed crucial for hospitals to address. Here is the list of the top 10 technology hazards;
Device-related hazards can lead to medical malpractice. In its 2015 top 10 Health Technology hazards, ECRI Institute lists 10 safety topics deemed crucial for hospitals to address. Here is the list of the top 10 technology hazards; 